Results of Erchonia’s Lunula Laser’s Clinical Trial for the Noninvasive Treatment of Nail Fungus Published in Podiatry Review
Erchonia today announces that the preliminary results of an ongoing clinical trial testing its Lunula laser for the noninvasive treatment of nail fungus have been published in the latest issue of the medical journal Podiatry Review.
“I am pleased to say the current data we are obtaining substantiates the Lunula laser as a safe and effective treatment for onychomycosis. Furthermore, the data demonstrates that Lunula is effective at treating varying degrees of infection.”
Erchonia today announces that the preliminary results of an ongoing clinical trial testing its Lunula laser for the noninvasive treatment of nail fungus have been published in the latest issue of the medical journal Podiatry Review (Vol. 71 No 2 pgs 6-9).
The technical article entitled, “Erchonia Laser Therapy in the Treatment of Onychomycosis,” summarizes the 48-week interim results of an 18-month study in which data for 323 patients has already been successfully recorded.
According to the results of the study, the Lunula laser validated percentages of nail clearance, specific to varying levels of nail inclusion. Post 4 treatments, the fungal infection was eradicated on 99% of study participants, and clear nail growth was also recorded on all these participants. It was also observed at the 48 week stage that only 4 patients were found to have suffered re-infection.
Unlike conventional hot lasers used for the treatment of fungal nail infections, the Lunula laser is reported to cause no pain to the patient and no temperature change in the treated area. Further results will be published at the close of the study.
Erchonia’s Lunula low level laser therapy is administered non-invasively and efficiently in just four treatments. Using low level laser light, it effectively eradicates fungus from the nail, nail bed and surrounding tissue — without pain and without adverse effects. All ten toes are treated at the same time in a 24-minute treatment unlike more conventional lasers where each toe is treated individually.
The primary author of the study, Robert Sullivan, states, “After reading the existing study data presented to me by Erchonia, I initiated a new, independent study to scientifically question this data. I am pleased to say the current data we are obtaining substantiates the Lunula laser as a safe and effective treatment for onychomycosis. Furthermore, the data demonstrates that Lunula is effective at treating varying degrees of infection. The successful treatment of nails with an initial percent involvement of up to 100% demonstrates the laser’s ability to permeate down to the nail bed.”
“This latest independent study validating the Lunula technology as a new standard to the safe and effective treatment for onychomycosis is a real breakthrough,” says Charlie Shanks, vice president of Erchonia Corp. “We are pleased to be able to share this data with the members of the Institute of Chiropodists and Podiatrists through their Podiatry Review publication and strive to carry on pushing the boundaries of low level laser therapy treatments.”
For more information, please visit https://www.erchonia.com.
About Erchonia
Erchonia is the global leader in low level laser healthcare applications. Over the last 15 years, Erchonia has been conducting research and development with the world’s leading physicians to advance the science of low level lasers. Prior to market introduction, all Erchonia lasers are proven safe and effective through independent level 1 clinical trials. Erchonia has garnered eight FDA 510 (k) market clearances and has several other products in research and development for new applications. Currently thousands of Erchonia’s lasers are used daily to reduce body fat, eliminate pain, and treat acne. For additional information, visithttps://www.erchonia.com.
Erchonia XLR8 Laser Granted FDA Clearance for Pain Treatment
Erchonia XLR8 Laser Granted FDA Clearance for Pain Treatment
 Depending on the settings selected, the Erchonia XLR8 laser can provide temporary relief of chronic neck and shoulder pain; reduce pain after liposuction of the thighs, hips and stomach; or reduce post-surgery pain after breast augmentation.
Depending on the settings selected, the Erchonia XLR8 laser can provide temporary relief of chronic neck and shoulder pain; reduce pain after liposuction of the thighs, hips and stomach; or reduce post-surgery pain after breast augmentation.About Erchonia
Harvard Scientists Commend Erchonia for Excellence in Clinical Research for the Zerona Laser
Erchonia is the global leader in low level laser healthcare applications. Over the last 15 years, Erchonia has been conducting research and development with the world’s leading physicians to advance the science of low level lasers. Prior to market introduction, all Erchonia lasers are proven safe and effective through independent clinical trials. Currently thousands of Erchonia’s lasers are used daily to reduce body fat, eliminate pain, and treat acne. For additional information, visithttps://www.erchonia.com.
Read more at http://www.virtual-strategy.com/2013/08/06/harvard-scientists-commend-erchonia-excellence-clinical-research-zerona-laser#kL8HuUUHbiGG4Ooy.99
New Clinical Trial Proves Erchonia’s Verjú Laser System Safely and Effectively Improves the Appearance of Cellulite

Erchonia is the global leader in low level laser healthcare applications. Over the last 15 years Erchonia has been conducting research and development with the world’s leading physicians to advance the science of low level lasers. Prior to market introduction, all Erchonia lasers are proven safe and effective through independent clinical trials. Currently thousands of Erchonia’s lasers are used daily to reduce body fat, eliminate pain, and treat acne. For additional information, visitwww.erchonia.com
Erchonia Wins Philanthropy Award from Parker Seminars

Erchonia Wins Philanthropy Award from Parker Seminars
Erchonia Chooses Zerona Model for 2012 Marketing Campaign
Amber Pohl Wins Contest, a Trip to Miami and $5,000 cash
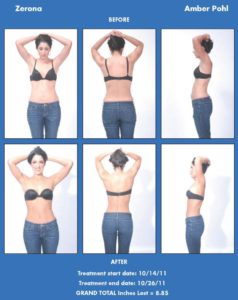
Erchonia Launches Zerona Model Search
Model Wins a Trip to Florida, $5,000 cash and Stars in Zerona’s 2012 Marketing Campaig
Erchonia, the global leader in low level laser healthcare applications, has launched a “Zerona Model Search” contest to find the next face—and body—of Erchonia’s 2012 marketing campaign for the FDA-approved Zerona non-invasive, painless, body contouring laser.
Erchonia is searching for someone who used the Zerona procedure to reduce inches from his or her waist, hips and thighs and would be interested in sharing the experience with others. Erchonia will award the winner a cash prize of $5,000, plus a four-night vacation to Miami, Florida, including airfare and hotel stay for two. The winner will also be photographed in a professional photo shoot and will be featured in Erchonia’s marketing campaign for one year.
To enter, visit Zerona’s Facebook page (www.Facebook.com/myzerona) to submit your name, address, phone number, a photo of you both before and after your Zerona procedure, what practice treated you, how many inches you lost with Zerona, and why you deserve to win. Submissions must be received by December 31, 2011.
“We’ve been so inspired by stories we’ve heard about how the Zerona procedure has really changed people’s lives, we thought this contest would be a fun way to learn more and reward people for accomplishing their goals,” says Charlie Shanks, vice president of Erchonia.
“Although we are only looking for someone who actually went through the Zerona procedure, the winner might not necessarily be the person who lost the most inches total. We are really interested in finding someone with a compelling story who encompasses the excitement, substance and sophistication of the brand,” adds Shanks.
Using low level laser light, the Zerona laser creates a pore in fat cells and stimulates fat to leak out of the cell. The cell deflates, but is not destroyed, and the fat is naturally processed by the lymphatic system as waste. After six treatments, one every other day for two weeks, patients lose an average of 3.65 inches from their waists, hips and thighs without pain, side effects or downtime. Zerona was approved by the FDA for non-invasive circumferential reduction based on a recent double-blind, placebo-controlled, multi-site clinical trial.
For more information, please visit www.myzerona.com. Follow Zerona on Twitter at www.Twitter.com/myzeronaand on Facebook, www.Facebook.com/myzerona.
About Erchonia
Erchonia is the global leader in low level laser healthcare applications. Over the last 15 years Erchonia has been conducting research and development with the world’s leading physicians to advance the science of low level lasers. Prior to market introduction, all Erchonia lasers are proven safe and effective through independent clinical trials. Currently thousands of Erchonia’s lasers are used daily to reduce body fat, eliminate pain, accelerate healing, and treat acne. For additional information, visitwww.erchonia.com.
Susan Woods, Crier Communications, 310-274-1072 x 202 susan@crierpr.com
Lose Love Handles with Zerona Laser myFOXla.com
Dermotologist Dr. Robert Seltzer says the non-invasive Zerona Lasers are cool to the skin, so there’s no topical anesthesia needed. In fact, he says the patient feels little to no pain, which means no downtime or recovery.Michael Brownlee has more on the procedure in this video report.
Dr. Robert Seltzer
960 E Green St.
Pasadena, CA 91106
(626) 449-3830
FDA Grants Market Clearance for Erchonia’s Zerona Laser

FDA Grants Market Clearance for Erchonia’s Zerona Laser
Proven safe and effective for circumference reduction of the waist, hips and thighs
About Erchonia
Susan Woods | Crier Communications | 310.274.1072 x 202 | susan@crierpr.com
Erchonia Body Contouring Clinical Trial
Erchonia’s Body Contouring Clinical Trial Published in Lasers in Surgery and Medicine
Erchonia, the leading developer of non-invasive, low level laser healthcare applications, announces today the results of its body contouring clinical trial are being published in Lasers in Surgery and Medicine’s January issue.
Lasers in Surgery and Medicine, the most widely-circulated, peer-reviewed scientific journal dedicated to laser therapy and diagnosis, is publishing the complete results of Erchonia’s recent, double blind, randomized, multi-site and placebo-controlled study. The study revealed that in six treatments with Erchonia’s Zerona laser – without surgery, diet restrictions or any other adjuncts – patients lost an average of 3.65 inches from their waists, hips and thighs.
Results using the Erchonia laser also have been recently published in the Plastic and Reconstructive Surgery, The Aesthetic Surgery Journal, The American Academy of Cosmetic Surgery Journal, and Clinics in Plastic Surgery and Liposuction.
“With this study, Erchonia has set the standard for non-invasive body contouring devices,” comments Steve Shanks, president of Erchonia. “We have been researching the Erchonia laser’s effect on fat cells for about 12 years. This inclusion in the world’s most prestigious laser journal ads even more credibility to our proven science.”
In contrast to high-power, high-heat lasers that are used in various surgical procedures, Erchonia lasers produce a low-level, or cold, output that has no thermal effect on the body’s tissue. Instead, the non-invasive laser helps the body absorb fat by stimulating biological function. As fat congregates in interstitial spaces of cells for a certain amount of time, the Erchonia laser stimulates the body to rid itself of that excess fat using the body’s normal processes.
About the clinical trial: Placebo patients were treated with a 635nm L.E.D. The test group was treated with the Erchonia’s Zerona laser. All patients were treated six times over two weeks, for twenty minutes on each side of the body (forty minutes total). Patients were instructed not to restrict their diets. Patients did not feel pain during the non-invasive treatment, did not receive anesthesia, and assumed normal activities after each treatment.
Erchonia pre-submitted the study results to the FDA for comments and set the clinical significance as a reduction of a minimum of 3 inches cumulative of the waist, hips and thighs. The FDA wanted to distinguish between statistically significant and clinically meaningful results which it felt would help with consumer confidence. Erchonia also submitted a 20-patient study on lipid panel reductions during this treatment as a safety measurement. The results of this study will be published at a later date.
The article published in Lasers in Surgery and Medicine represents all of the data that Erchonia submitted to the FDA for market clearance or 510k which Erchonia expects is imminent.
About Erchonia
Erchonia is the global leader in low level laser healthcare applications. Over the last 15 years, Erchonia has been conducting research & development with the world’s leading physicians to advance the science of low level lasers. Prior to market introduction, all Erchonia lasers are proven to be safe and effective through independent clinical trials. Currently thousands of Erchonia’s lasers are used daily to reduce body fat, eliminate pain, accelerate healing, and treat acne. For additional information, visit www.erchonia.com
Contact: Katie Cycan, Crier Communications, 310-274-1072 x 207 katie@crierpr.com
Susan Woods, Crier Communications, 310-274-1072 x 202 susan@crierpr.com
