5 Exercise Routines You Can Do at Home
Are you trying to have a healthy and fit summer? On the road with business or family? Or maybe you’re just too shy or embarrassed to exercise in public right now? There are ways that you can still get a good, effective work out right in the privacy of your home or hotel room. We’re going to look at 5 exercise routines you can do without fancy equipment or a trip to the gym.
1.) Jumping rope – You can find a jump rope for a buck or two at the local dollar store. Put it to use for 20 minutes of skipping, jumping fun and you can burn more than 200 calories. Find your favorite sit-com on Netflix and skip while you watch to make the time go faster.
2.) Surya Namaskar – The hatha yoga sun salutation is a perfect workout. It is a series of 12 poses done fluidly and gently. Begin with 5 rounds, working your way up to 25 minutes or so. Doing so will burn 350+ calories. One caution—do it on an empty stomach. The Surya Namaskar was designed to be done in the early morning, upon waking, before breakfast. You don’t want to be doing inverted poses on a full tummy.
3.) Calisthenics – Just because they are old fashioned doesn’t mean the calisthenics you learned in school aren’t good for you. Try the following series:
a. 50 jumping jacks
b. 15 crunches
c. 10 tricep dips: use a chair or bench or even the sofa
d. 5 push-ups
e. 10 squats
f. 10 lunges (each leg)
g. 10 calf raises
h. 10 toe touches
i. 30-second plank
This will work every major muscle group, give you a good all over work out, and burn a lot of calories. Be sure to stretch before and after to serve as both warm up and cool down.
4.) High March – This is a quick exercise that you can use while waiting for your tea water to boil or for your television program to resume during a commercial break. Simply stand up and march in place, lifting your knees all the way up to your waist. As you bring up each knee, either raise the opposite arm until the upper arm is parallel with the floor, or bring the opposite fist to rest on the raised knee. Keep this up for a solid 60 seconds and you’ll tighten and tone core muscles, thighs, glutes, upper back, chest, and arms. Not to mention you’ll burn a bunch of calories. Not bad for a minute of your time, huh?
5.) Plank Crawls – The plank is a great exercise because it works so many major muscle groups. A plank crawl is a variation that some find more enjoyable than just planking, and some experts say is actually more beneficial than the traditional static plank. Begin in a raised push-up position. Lower ONE arm to the floor in a plank position. Then, lower the other arm. Hold for one breath. Then, beginning with the first arm lowered, raise yourself, one arm at a time, to the raised push up position. Do 15 to 20 complete crawls for a good, effective workout. You can lower your knees, if need be, to reduce some of the intensity of the exercise.
Just because you can’t or don’t want to get to the gym doesn’t mean you can’t work on losing weight, toning up, and looking and feeling great. Get that workout gear on and hit the living room!
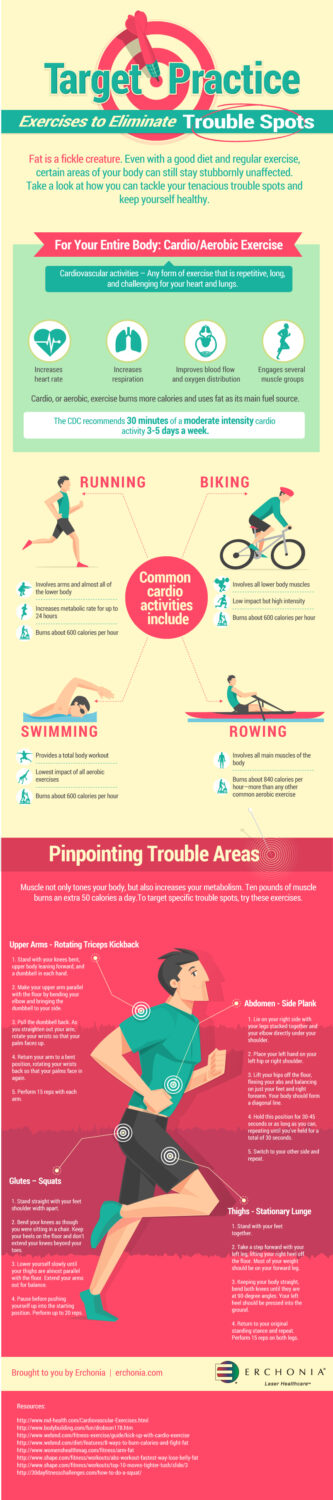
Exercises to Eliminate Trouble Spots
Fat is a fickle creature. Even with good diet and regular exercise, certain areas of your body can still stay stubbornly unaffected. Take a look at how you can tackle your tenacious trouble spots and keep yourself healthy and fit.
Recipe: Super Summer Slim Down Smoothie
While summer’s almost over, it’s not over yet. What better way to enjoy the last weeks of the season than with a delicious smoothie that celebrates all the flavors of the season and aids in your efforts to stay healthy & fit this summer.
This super smoothie recipe will cool you off, give your body lots of good vitamins and antioxidants, and keep your slim-down going strong. Oh, and it doesn’t hurt that it’s awesomely delicious, either!
Note: This smoothie is filling and nutritious enough to serve as a meal replacement for breakfast or lunch. In other words, don’t add this one to your diet meal plan as an afternoon or evening snack, or you might just counteract the slimming effect of the smoothie.
Super Summer Slim-Down Smoothie
½ medium banana (ripe)
1 cup frozen mixed berries
¼ cup low-fat Greek yogurt (get plain for no added sugar)
¾ cup milk (soy, almond, skim, rice—your choice)
2 tablespoons dark chocolate chips
Optional: 1 tsp. pure vanilla extract
Chuck everything into your blender and give it a whirl until smooth and well-mixed. If you need to thin it, add 3 to 5 ice cubes. It makes 1 large glass of delicious, cold, refreshing smoothie.
How Does This Smoothie Help Your Slim-Down Efforts?
Bananas are filling. They also add a natural sweetness, but are bland enough to mix well with just about any flavor. Their texture helps with the creamy consistency. And they are a natural source of energy. They provide a steady stream of energy, enough to get you through a 45-minute workout or that after-lunch slump that hits like a ton of bricks every afternoon. Unlike caffeine or sugar, there’s no crash or hard comedown from a banana high, either. Let’s not forget that some of this energy comes from carbs, but a great deal of it comes from all the vitamin A, iron, phosphorous and potassium that bananas contain.
Berries are filled with antioxidants. They are also naturally sweet and high in water content. Including a mix of berries ensures that your smoothie will be packed with vitamin C and phytochemicals of all sorts. They also add some fiber to your smoothie, helping you to keep feeling full longer. Plus, fresh berries are the ultimate summertime treat.
Greek yogurt is a great low-fat, low-carb source of protein. It’s also filled with bone-strengthening calcium and belly-friendly probiotics. Greek yogurt has been shown to aid in weight loss, and can help reduce bloating and gas.
Whether you choose soy milk, almond milk, or just ordinary skim milk, adding milk to your smoothies is another great way to add tummy-pleasing protein and calcium. You need protein to stave off hunger pangs longer.
Monounsaturated fatty acids (MUFAs) have been shown to be vital in weight loss efforts, especially in fighting belly and midriff fat. Sources of MUFAs are dark chocolate, nuts and nut butters, flaxseed oil, and avocado. Adding some dark chocolate chips to your slimdown smoothie aids you in your slimming efforts, making your smoothie just that more effective in doing what you want it to do. Plus, this ingredient (and the optional pure vanilla extract) makes this smoothie taste like a treat—without any of the guilt.
Erchonia Launches Marketing Library for Customers
The Erchonia Marketing Portal is designed to assist doctors & practitioners with promoting their Erchonia devices. We offer materials for purchase or for download that can be used “as is”, these are print ready files. The print ready files can be personalized with your office information. We also offer for download Erchonia product pictures & logos to create your own material. All use of the Erchonia Marketing Portal materials is governed by the Terms of Use. Our Terms of Use available at www.erchonia.com/terms-of-use .
How 3LT Can Help Our Equine Friend
Humans domesticated horses millennia ago, changing the way our ancestors traveled, fought, and survived. They are majestic, beautiful creatures built for speed and power.
Sadly, one of the most serious and devastating diseases affecting horses, ponies, and other equine animals is laminitis. Let’s take a closer look at laminitis and how low-level laser therapy can give our equine companions a leg up.
What is Laminitis?
Laminitis describes a condition wherein the laminae—the tissues bonding the hoof wall to the pedal bone in a horse’s hoof—become weakened and inflamed from disruptions in blood flow, leading to tears in the structure supporting the pedal bone within the hoof. Laminitis typically occurs in a horse’s front feet. The condition is caused by various physical and metabolic issues, including:
- An excessive intake of grain or grass
- High levels of insulin
- Enlargement of the pars intermedia in the pituitary gland
- Impact from riding on hard surfaces
- Stress from long distance travel
This can result in tremendous pain, lameness, and deterioration in the hoof. Left untreated, laminitis can cause the pedal bone to rotate and point downwards. In worst cases, the pedal bone will penetrate through the hoof wall.
Laminitis greatly reduces a horse’s usefulness, and many horse owners are forced to put down the horse to prevent further suffering.
Treating Laminitis
Many traditional treatments are expensive and time-consuming and don’t guarantee full recovery. These include changing your horse’s diet, providing greater hoof care, and moving your horse to a different enclosure featuring deep shavings or sand. Severe cases wherein the pedal bone has sunken through the hoof require surgical procedures involving tendon release, but this can put the horse at risk of infection or cause damage to surrounding structures.
Low-level laser therapy has been used in humans to treat joint pain, edema, soreness, and wounds, but veterinarians have extended these laser treatments to horses suffering from laminitis. Studies show that the photon energy in a low-level laser stimulates blood vessels in a horse’s foot, promoting greater circulation, better tissue nutrition, and ultimately faster healing. Laser therapy also greatly reduces the chance of infection or damage to surrounding areas as the procedure is entirely non-invasive.
Animal Health Options, a purveyor of innovative and effective supplements for animal wellness since 1990, has found success in incorporating low-level laser therapy into its treatment for laminitis. Horses undergo low-level laser sessions two to three times a week. This is coupled with:
- A restrictive diet to reduce weight and make up for insulin resistance
- Plenty of lying down to keep pressure off the affected feet
- Visits with a farrier to trim hooves to correct the angle of the feet
Preventing Laminitis
One of the best ways to treat laminitis is to prevent it from happening altogether. While you can’t always predict your horse’s health, you can control parts of his environment, primarily his diet. Too much grain or lush green grass leads to excessive sugars stored in the hind gut. When these sugars are absorbed, the horse develops hyperinsulinemia (an overload of insulin), which can trigger laminitis. A bad diet can also lead to obesity, putting more pressure on your horse’s hooves. To keep your horse’s diet in check:
- Feed your horse a high fiber, forage-based diet, comprising a mixture of mature grass, hay, and alfalfa. Vegetable oils can be included in this diet for added calories.
- Carefully manage grazing. Considering grazing your horse at night, when sugar levels are lowest.
- Avoid hard feed unless your horses are performing hard work.
Maintain a regular hoof trimming schedule for good hoof health. Your horse may also need specialist shoeing for proper support.
The Skinny on Fat-Fighting Foods: 6 Foods to Help You Lose Weight
People are under the common misconception that staying healthy means eating less. Far from causing fat loss, skipping meals actually causes numerous health problems, including:
- A slowed-down metabolism
- Deteriorating muscles
- General fatigue
- Impaired concentration
Contrary to what you think, eating can help you lose weight. It’s just a matter of finding the right foods to eat. Let’s take a look at some foods that can help you shed pounds and improve your health.
- Eggs
Doctors, dieticians, and scientists seem to have seasonal debates about eggs and their validity in the nutrition realm, particularly in the area of cholesterol, but you can rest easy. In fact, eggs are good for you and can help you stay thin. One study published by the International Journal of Obesity found that participants who had eaten two eggs for breakfast for eight weeks lost 65% more weight than those who ate bagels for breakfast.
While most dieters eat just the egg whites, the yolks contain half the protein of an egg—and a lot of the nutrients. The high protein content is what makes eggs so great. One large egg contains about six grams of protein and nine amino acids with only 77 calories and five grams of fat. That keeps you satisfied for longer and prevents unhealthy snacking. The proteins also stimulate the release of glucagon, which aids in metabolism and can help burn belly fat.
Along with protein, eggs are rich in:
- Vitamins A, B12, and C
- Iron
- Phosphorous
- Selenium
- Almonds
Almonds are high-quality nuts that are rich in vitamin E and monounsaturated fats—the good kind of fat. Almonds are packed with fiber, keeping you full without adding extra calories. They contain magnesium and vitamin B2, which can combat stress, calm nerves, and boost your energy levels. Almonds also contain zinc to curb sugar cravings and oleic oils to curb hunger pangs. If that’s not enough, almonds are also good for your skin and hair.
Almonds also happen to be easy to include in your diet. Snack on a handful at your desk or sprinkle chopped almonds on your meal for added crunch and flavor.
- Chia Seeds
If you’re looking for fiber, you needn’t look any further. Chia seeds are extremely high in soluble fiber. This prevents the absorption of fat, lowers cholesterol, and stabilizes blood sugar levels. The high fiber acts like a sponge, allowing chia seeds to soak up to twenty times their weight in sugar and liquids. As the chia seeds plump up, they expand in your stomach, keeping you full for longer without the extra calories. They are also rich in calcium, iron, and omega-3 fatty acids.
Add chia seeds to your oatmeal or create a healthy pudding of chia seeds and unsweetened chocolate almond milk.
- Quinoa
Although it’s a grain, quinoa acts more like a vegetable and is a close relative of kale, spinach, and Swiss chard. High in fiber and protein, quinoa offers a sturdy foundation for healthy fullness while also offering plenty of vitamins and minerals, including iron and vitamin B12. Quinoa has a low glycemic index, so it won’t cause spikes in blood sugar. One serving of cooked quinoa only has about 172 calories.
The best part about quinoa: you can eat it with just about anything. Eat it plain, add it to veggies, salads, or consume with cinnamon, banana, and almond flakes for an oatmeal replacement. The possibilities with this gluten-free grain are endless.
- Grapefruit
Sweet and tangy, grapefruit is a great source of vitamin C and an antioxidant called lycopene, but these treats may also offer a helping hand in weight loss. One study conducted on mice found that daily grapefruit juice intake expedited weight loss, improved blood glucose, and controlled insulin levels.
Some studies suggest that the mere smell of grapefruit is enough to aid in weight loss, but feel free to go in for the real thing, raw or juiced.
- Yogurt
Yogurt offers a healthy dose of calcium, vitamin D, and protein to keep you full, but probiotics are the key fat-burning ingredient in yogurt. Consuming active bacteria may seem unpleasant, but probiotics are the type of germs that your body needs. They reduce the amount of fat your body absorbs and add flora to your gut that improve your digestion. Eat yogurt plain, add fruit, or use it for creamy sauces. Opt for an unsweetened Greek yogurt instead of regular if you can—Greek yogurt has almost twice the protein and fewer carbs and sodium than regular yogurt.
Food gives you the energy you need to live life to the fullest. Instead of depriving yourself, learn to eat the right things to keep your body healthy and fit. What are some of your favorite foods for weight loss?
Sources:
http://www.fitnessmagazine.com/weight-loss/eating/weight-loss-foods/
http://www.ahealthiermichigan.org/2014/08/23/two-eggs-in-the-morning-can-help-you-lose-weight/
http://metro.co.uk/2013/10/30/how-almonds-can-help-you-to-lose-weight-4163723/
http://www.doctoroz.com/slideshow/5-ways-chia-can-change-your-life?gallery=true
http://www.fitday.com/fitness-articles/fitness/weight-loss/4-reasons-the-quinoa-grain-can-help-you-lose-weight.html#b
http://www.popsugar.com/fitness/Ways-Grapefruit-Can-Help-You-Lose-Weight-26331222
http://www.fitday.com/fitness-articles/fitness/yogurt-smackdown-greek-vs-regular.html#b
Curb Your Cravings: Your Will Power and Eating Smart This Holiday Season
The holiday season can wreak havoc on your diet. From signature apple pies to decadent cookies and creamy mashed potatoes, the environment is fraught with traps that seem set up just to derail your resolve and have you elbow deep in a pile of irresistible holiday treats. But before you give up altogether, consider trying to adhere to the following smart tips that can help you lower your chance of disaster while maintaining a healthy dose of cheer.
Let Yourself Indulge…Later
This tip might initially seem counterintuitive, but studies show that individuals who completely deprive themselves of their edible desires often buckle and end up eating even more than those who allow themselves to indulge. Interestingly enough, those who allow themselves their food of choice, but not until later, either ended up eating a more moderate amount or none at all. Giving yourself permission to indulge but making yourself wait buys you time to allow the craving to potentially pass. Plus, if you eat something more nutritious first, you’ll take a lot of the power out of that craving and find that it’s not begging quite so loudly anymore.
Stick to Your Exercise Routine
Maintaining your gym routine throughout the holidays can remind you how hard you’ve worked to maintain your goal up until this point. It can also help you curb cravings and improve your mood. As an added benefit, it also allows you to burn off more calories—offering you some much-needed breathing room in your daily calorie count.
Set a Time Limit
The holidays are a time of abundance, and leftovers, snacks, and baked goods are often no more than an arm’s reach away. By setting certain times during the day that are dedicated to eating, you can prevent yourself from the constant grazing that can pack on pounds. By limiting not only the times during the day which are dedicated to eating, but also the number of snacks per day, you can set concrete barriers that can keep your consumption from running wild. Of course, if you find yourself genuinely hungry during an off time, be sure to grab a healthy snack to keep your blood sugar at a healthy level—a handful of raw nuts, an apple, or some Greek yogurt will do the trick.
Limit Your Variety
When we have a wide variety of foods in front of us, we tend to eat more. One deviled egg here, one cookie there, a dollop of mashed potatoes and a few candied yams may not seem like much when you look at each food separately, but combined they can add up fast. By limiting the number of foods you consume, you can prevent your mind from tricking you into thinking you’ve only had a few bites here and there. The holidays are a time for enjoying festivities with loved ones, and that almost always includes delicious food and drink. Have fun and partake in the holiday cheer, but remember these tips to keep your health and fitness routine on track.
3 Myths (and Truths) about Fat Loss
Every new day brings with it a new way to lose weight. But many of these new diets perpetuate pervasive myths about just how fat loss occurs and what can help an individual shed unwanted pounds. Here we’ll take a look at some of the most common fat loss myths and focus on tried-and-true ways to successfully shed that winter layer.
Myth: Saturated fat increases bad cholesterol and the risk of stroke and heart disease. Saturated fat has long been demonized, and its role in climbing cholesterol numbers has been focused on the LDL, or “bad” form of cholesterol. But the fact of the matter is that eating saturated fat also increases HLD, or “good” cholesterol as well! This means that saturated fat effectively has no effect on bad vs. good cholesterol numbers, as it increases both in tandem. Although a common claim, modern-day research has yet to prove an association with ingestion of saturated fat and an increase in the risk of an individual suffering from stroke, cardiovascular heart disease, or coronary heart disease.
Myth: A high carbohydrate diet is better than a high fat diet. Everyone knows that eating fat is always bad, right? Well, it turns out that is not necessarily true. Studies have shown that fat releases hormones that increase satiety levels, lowering an appetite that might otherwise be spurred by an overabundance of high carbohydrate foods. Oils and healthy dietary fats such as those found in avocados take time to break down, helping normalize insulin levels within the blood. Carbohydrates, on the other hand, can spike blood sugar and then insulin, creating drastic swings in glucose levels. Research concludes that an overabundance of carbohydrates—not fats—is linked to weight gain and Type 2 diabetes.
Myth: Eating at night will make you gain weight Everyone has heard this one. If you eat at night, your body will store that late night feast as fat. Many diets have strict rules about not eating after eight or nine and to lock yourself away from the snack cabinet. But likely the reason that those who eat late at night tend to gain weight is simply that they are consuming more calories throughout the day than those who do not. Although there is no definitive proof to determine whether time of day counts, the total amount of calories consumed is the number one contributor to weight gain or weight loss. The better rule of thumb is to eat when you’re hungry, not according to a clock, and stop eating a bit before you feel full.
When it comes to weight loss, health, and fitness, rumors and misinformation run rampant. Be sure to put in some research to ensure that you’re giving your body the nutrients it needs to thrive!
The Layman’s Guide to Plantar Fasciitis and Its Treatments
What you recognize as the sudden onset of an intense pain in your heel, Mayo Clinic staff refers to as plantar fasciitis. It denotes an inflammation of the plantar tendon, which is a layer of tissue that stretches across the bottom of your foot. The plantar fascia connects the bone in your heel with your toes and presents sharp stabs of pain when inflamed.
What Causes Plantar Fasciitis?
Long periods of standing, running downhill, wearing high-heeled shoes, and climbing stairs may cause the condition. In fact, any movement that causes a pull on the plantar tendon has the potential to result in painful stabbing sensations in the heel. For runners in particular, plantar fasciitis creates a problem when their running styles incorporate a consistent heel strike. Ballet dancers and high-impact Zumba participants, too, may find that these exercises overstress the tendon.
Other factors leading to the condition include obesity and footwear that does not provide adequate support for the feet. Sports hobbyists do not always recognize that the internal support system in their shoes has given way long before the shoes look like they need to be replaced. By delaying the replacement of the footwear, these folks run the risk of encouraging calcium buildups near the heel, which results in consistent pain when exercising, walking, or even standing.
Counteracting Early Warning Signs
The good news is that plantar fasciitis does not appear overnight. The condition builds up gradually. At the first sign of pain on the bottom of your foot or an uncomfortable feeling in the heel, it is time to take counter measures.
- Footwear. Stop wearing unhealthy footwear. Turn to shoes with proper arch support and replace your running or exercise shoes regularly.
- Change your running style. Runners should examine their foot landing styles. If they realize that they are landing on their heels, it is crucial to make a conscious effort to change the landing pattern to a healthier fore-heel strike.
- Change your running locations. Although running hills offers you more fitness, the pain in your heel calls for flat surface running only.
- Reduce high-impact activities. Consider switching out your dance aerobics routine for water aerobics when you feel the first twinges of pain.
- Cushion your feet. When you stand for long periods at work or at home, invest in cushioning mats that take some of the pressure off your heel. These mats reduce the stress your plantar fascia experiences.
Dealing with Fully Developed Plantar Fasciitis
When the early twinges of pain have developed into a chronic condition that causes pain every time your foot touches the ground, you still have options. It starts with a visit to your doctor. This professional helps to rule out other problems.
Over-the-counter pain relief medication can ease the pain. The downside is the potential for side effects when you take these medications for longer periods. Since plantar fasciitis may last for a while, discuss the use of these medications and their side effects with your doctor before making this decision. Some physicians suggest the use of steroid injections, which do have the potential of greatly reducing the pain. Unfortunately, this treatment can cause more problems than it solves when used repeatedly.
Consult with a physical therapist about the best way that you can change your gait to undo the inflammation now and prevent it from recurring in the future. Orthotics assist with day-to-day activities such as standing at work or walking to your car. Experts at Erchonia have had excellent success with low-level laser therapy. After administering two 10-minute therapeutic laser treatments per week to the heels of affected patients over the course of three weeks, the majority of patients reported a positive change in pain levels. Whereas over-the-counter medications and steroid injections do bear some risks, the laser method did not lead to the report of any side effects.
[INFOGRAPHIC] With Laser Focus: What You Should Know Before Undergoing Laser Body Contouring
Laser body contouring remains one of the most advance medical procedures today, promoting natural fat loss through a non-invasive process. While non-invasive means you won’t have to woryr about incisions or anesthesia, it doesn’t necessarily mean the procedure is risk-free. Stay safe by doing your research.