
First and Only FDA Market Cleared Low-Level Laser Treatment Option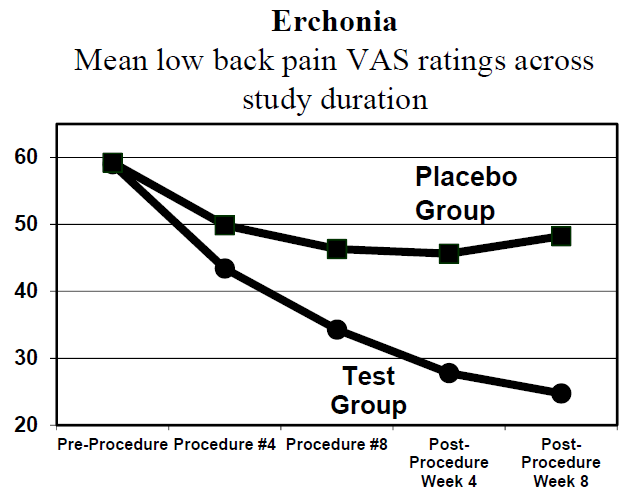
Melbourne, FL (PRWEB) July 17, 2018
Erchonia Corporation announces another successful clinical trial that has resulted in the granting of an FDA 510(k) market clearance for chronic low back pain of musculoskeletal origin. This grants another first for Erchonia, which started the low-level laser category in January 2002. That year, the FDA issued its first 510(k) market clearance for any low-level laser based on Erchonia’s clinical trial for chronic neck and shoulder pain.
This new indication for chronic low back pain is the only laser to be market cleared by the FDA. Erchonia’s long history and dedication to the science of low-level laser therapy has led to 22 FDA 510(k) market clearances based on their patented laser systems.
About the study: Erchonia through the pre-IDE process worked with the U.S. FDA on study design and success criteria. The success criteria were defined as a minimum of a 30% decrease in chronic low back pain and 35% of patients in the treated group would experience the minimum pain reduction compared to the placebo group. Overall, 72% of patients met the success criteria. Steven Shanks, President of Erchonia stated, “We believe we have demonstrated that the use of non-thermal lasers has proven to be a far better option for treating low back pain than that of opioids or NSAIDS.”
A recent study published in JAMA in 2018 titled The Space Randomized Clinical Trial looked at chronic low back pain with opioids and NSAIDS over 1 year. Opioids demonstrated a 30% reduction in pain and NSAIDS proved a 34.5% reduction in pain. The publication concluded that “Results do not support initiation of opioid therapy for moderate to severe back pain or hip or knee osteo arthritis pain”.
Taking into consideration the minimal effectiveness (30%) and the opioid crisis along with the side effects of NSAIDS for chronic low back pain, doctors and patients may now have safer, more effective option for chronic low back pain of musculoskeletal origin that has been proven successful with no side effects or adverse events.
Erchonia would like to thank doctors Greg Roche, Trevor Berry and Paul Quarneri for their dedication to the science of low-level laser therapy and for helping them document this placebo-controlled, randomized, double-blind, parallel group, multi-center clinical study.
For more information, please visit http://www.erchonia.com.
About Erchonia
A small family company is changing the world with the most advanced non-invasive lasers on the market. Erchonia went from starting in a small garage in 1996 to selling their product in over 50 different countries around the world in 2018. Erchonia has been passionate about researching and developing low level lasers since the beginning with over (15) FDA clearances for treating chronic pain and promoting fat loss. As this family has grown so has the world of non-invasive drug-free healthcare solutions.

